May 19, 2025 | 21:38 GMT +7
May 19, 2025 | 21:38 GMT +7
Hotline: 0913.378.918
May 19, 2025 | 21:38 GMT +7
Hotline: 0913.378.918
Before Vietnam successfully produced a vaccine against African swine fever ('ASF'), the story of preventing this dangerous disease from spreading and causing significant damage was always a concern for livestock farmers worldwide.
According to Dr. Tran Xuan Hanh - Deputy General Director of Navetco Company- vaccines against ASF are challenging to research and produce for many reasons. First of all, viruses are large in size and complex in structure. There have also been many studies on the virus, the disease mechanism, and the epidemiology of the disease. However, the understanding of this virus is still minimal, especially on the immune mechanism in African swine fever.
In addition, this is a new vaccine, and there are very few documents for reference. The world does not even have a method to evaluate this vaccine. Therefore, besides being similar to the vaccine virus provided by IPADC in the United States, Navetco Company must research from the beginning to build production, storage, quality inspection, usage, etc. Even if provided the breed, when Navetco Company receives it, it must be re-evaluated to see if it is compatible with the strain of African swine fever virus that is causing disease in pigs in Vietnam. Also, because it is a new vaccine, the Company also had difficulty finding a location to test...
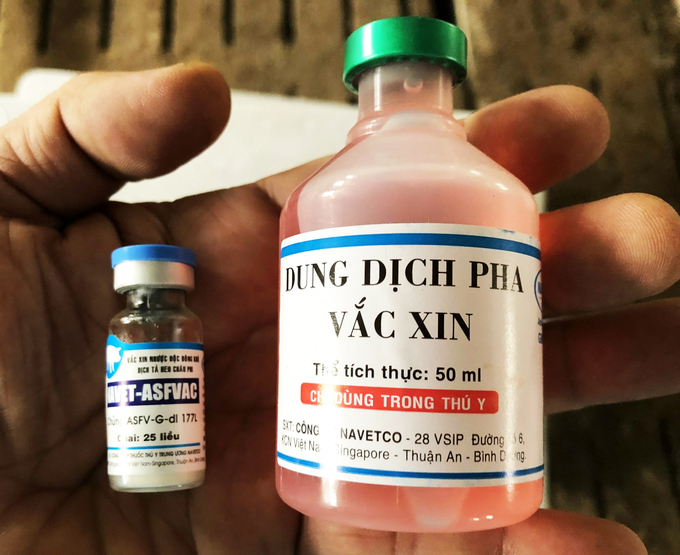
However, with capacity, determination and passion for science, Navetco Company finally launched a vaccine against ASF.
NAVET-ASFVAC vaccine results from cooperation between Navetco Company under the Ministry of Agriculture and Rural Development, Vietnam, and Plum Island Animal Disease Center (IPADC) under the Agricultural Research Institute, USA. With the consent of the Vietnam Department of Animal Health, the Company received the vaccine virus strain ASFV-G-delta-I 177L. It transferred technology from the Plum Island Animal Disease Center, USA. NAVET-ASFVAC vaccine has been licensed for circulation under registration number TWII-171 and certificate of circulation number 538/QLT-SX 22, dated May 18, 2022.
According to Dr. Tran Xuan Hanh, Navetco Company has undergone many stages, both in the laboratory and in extensive supervised experiments, to produce the best and most objective evaluation results for the vaccine against ASF.
Evaluation results of the African swine fever virus strain ASFV-G-delta-I 177 L show that this strain belongs to genotype II and has similar antigens to the African swine fever virus that causes disease in pigs in Vietnam. In other words, pigs vaccinated with this virus strain (ASFV-G-delta-I 177 L) will create immunity that can prevent African swine fever caused by the African swine fever virus circulating in the country.
Under laboratory conditions, the vaccine is safe when injected into pigs at 5-10 times the used dose. Using the ELISA method to test the antibody response 28 days after vaccination with NAVET-ASFVAC gave the results: pigs had an antibody response ranging from 85-96% and using the challenge method with the cholera virus strain. In virulent African pigs isolated in Vietnam, when testing the effectiveness of 6 consecutive vaccine batches, 100% of vaccinated pigs were protected after the virulent challenge, and 100% of unvaccinated pigs died. Currently, the Company has produced more than 13 batches of vaccines that meet quality requirements for the following criteria: sterility, purity, safety, and effectiveness. The Company also sent seven sets of vaccines to Central Veterinary Drug Testing Center 1 to check the quality, and the results of all batches were concluded to meet the requirements and were allowed to be used.

Vaccinate pigs with NAVET-ASFVAC vaccine.
After being granted a license to produce and circulate NAVET-ASFVAC vaccine (signed on May 18, 2022), implement the direction of the Ministry of Agriculture and Rural Development and the Department of Animal Health on supervised vaccination of NAVET-ASFVAC vaccine and disease prevention of African swine fever for pigs raised in our country, especially after the dispatch No. 4870/BNN-TY dated April 27, 2023 (Regarding the use of vaccines to prevent African swine fever sent to the Human Resources Committee (people of provinces and centrally run cities) the vaccination work has been carried out more smoothly and achieved positive results.
Thereby proving that the vaccine is safe and effective in protecting vaccinated pigs; through monitoring more than 125 thousand vaccinated animals, the rejection rate after vaccination is about 0.2%, but the cause's not because of the vaccine. Regarding disease prevention effectiveness assessed by ELISA, 85.5 - 97.5% of vaccinated pigs had good antibody responses. In particular, through testing of 500 fecal samples taken from vaccinated pig pens, the existence of the vaccine virus in the environment was not detected (results were carried out by the Department of Animal Health Region 6 and the Central Diagnostic Center).
Translated by Tuan Huy
![Reducing emissions from rice fields: [Part 1] Farming clean rice together](https://t.ex-cdn.com/nongnghiepmoitruong.vn/608w/files/news/2025/05/05/z6509661417740_a647202949c539012a959e841c03e1d3-nongnghiep-143611.jpg)
(VAN) Growing clean rice helps reduce environmental pollution while increasing income, allowing farmers to feel secure in production and remain committed to their fields for the long term.
/2025/05/19/5136-1-144800_230.jpg)
(VAN) The Nghe An Provincial People's Committee has just approved the list of beneficiaries eligible for revenue from the Emission Reductions Payment Agreement (ERPA) in the North Central region for the year 2025.

(VAN) 14 out of 35 domesticated elephants in Dak Lak province have had their living conditions improved, with 11 of them currently participating in the non-riding elephant tourism model.

(VAN) Muong Nhe Nature Reserve hopes that being upgraded to a national park will lay the foundation for forest protection efforts to be carried out in a systematic, modern, and sustainable manner.
/2025/05/16/3923-2-171845_52.jpg)
(VAN) Lower costs, higher yields, and improved soil quality are outstanding benefits that soybeans bring when integrated into the crop rotation system.

(VAN) The 'For a Green National Environment' programme aims to promote a green lifestyle, support businesses in implementing ESG practices, and turn Net Zero commitments into concrete actions.

(VAN) Cold-barn systems efficiently manage environmental and temperature conditions, which aids in the prevention of respiratory diseases in pigs and protects them from the vectors that transmit African swine fevers.